Preliminary study on fluctuation of the Bemisia tabaci (Hemiptera: Aleyrodidae) in greenhouse tomato and pepper crops, Tucumán, Argentina
Estudio preliminar sobre la fluctuación de Bemisia tabaci (Hemiptera: Aleyrodidae) en cultivos de tomate y pimiento bajo cubierta, Tucumán, Argentina
*Corresponding author: c_reguilon@yahoo.com.ar
Received: 12 October 2018 - Accepted: 27 February 2019
Abstract
The aim of this study was to determine the abundance and population fluctuations of Bemisia tabaci (Gennadius) in greenhouse tomato and pepper crops in Lules department, Tucumán province (Argentina). Entomological sampling was carried out from July 2008 to March 2009. Adults were collected using sticky traps while immature individuals were collected from the leaflets of the different plant strata. A total of 121.075 individuals of B. tabaci were collected, from which 12.630 corresponded to eggs, 8.718 to nymphs, 262 to pupae, and 99.465 to adults. In general terms, the abundance of B. tabaci increased considerably from the third week of sampling and stayed high, with pepper crops showing the highest number of individuals.
Keywords: abundance; pests insect; Solanum lycopersicum; Capsicum annum
Resumen
El objetivo del trabajo fue determinar la abundancia y fluctuación poblacional de Bemisia tabaci (Gennadius) en cultivos de tomate y pimiento bajo cubierta en el departamento Lules, provincia de Tucumán (Argentina). Los muestreos entomológicos se realizaron desde julio de 2008 a marzo de 2009, recolectándose adultos mediante trampas adhesivas e inmaduros en los foliolos de los diferentes estratos de las plantas. Se recolectó un total de 121,075 individuos de B. tabaci, de los cuales 12,630 corresponden al estado de huevo, 8,718 a ninfa, 262 a pupa y 99,465 a adulto. En líneas generales, la abundancia de B. tabaci aumentó considerablemente a partir de la tercera semana de muestreo y se mantuvo elevada, siendo el cultivo de pimiento el que presentó mayor número de individuos.
Palabras clave: mosca blanca; abundancia; insecto plaga; Solanum lycopersicum; Capsicum
Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) is among the most economically important pests in the world. In Latin America and the Caribbean, the whitefly has led to losses that have diminished the productivity of socioeconomically important crops, such as Ipomea batatas L. (sweet potato) (Convolvulaceae), Citrullus lanatus (Thunb.) Matsum & Nakai (watermelon), Cucumis melo L. (melon), Cucumis sativus L. (cucumber), Cucurbita maxima Duchesne (squash), Cucurbita argyrosperma Huber (cushaw pumpkin), Cucurbita moschata Duchesne (crookneck squash) (Cucurbitaceae); Glycine max L., (soybean) and Phaseolus vulgaris L. (bean) (Leguminosae); Gossypium hirsutum L. (cotton), Abelmoschus esculentus (L.) Moench (okra) (Malvaceae); Capsicum annum L. (pepper), Solanum lycopersicum L. (tomato), Solanum melongena L. (eggplant), Solanum tuberosum L. (potato) and Nicotiana tabacum L. (tobacco) (Solanaceae) (Brown, 1993; Byrne et al., 1990; Caballero and Pitty, 1995; Lourenção and Nagai, 1994).
Bemisia tabaci causes both direct and indirect damage to crops. It causes direct damage through the sucking of phloem sap causing the weakening of the plant, chlorosis, and foliage deformation (López-Ávila, 2005). And it also causes indirect damage due to the accumulation of excreted honeydew, which favors the development of sooty mold fungi, causing plant asphyxia, reduction of the photosynthesis process, and interference in the deposition of chemical products used for controlling whiteflies, ultimately leading to the loss of the commercial value of fruits (Vet et al., 1980; Llorens Climent and Garrido Vivas, 1992; Salguero, 1993 López-Ávila, 2005). However, the more serious damage is the transmission of bacterial and virus-borne diseases, the most important being the geminivirus. Among them the tomato yellow mosaic virus (TYMV) and the tomato yellow leaf curl virus (TYLCV) are the most relevant (Vet et al., 1980; Llorens Climent and Garrido Vivas, 1992; Salguero, 1993; García Marí et al., 1994; Naranjo et al., 2004; Polack, 2005).
In Argentina, B. tabaci was cited for the first time in 1943, on cotton crops in Chaco province (Mound and Halsey, 1978), and in 1955 it was registered in Tucumán province (Viscarret, 2000). However, in 1994 the first studies of the population dynamics of the white fly and its associated parasitoids in northern Argentine appeared (Viscarret, 2000), as well as studies of the presence of the geminivirus, associated to B. tabaci in soybean, tomato, bean and pepper crops (Mound and Halsey, 1978; Viscarret et al., 2001).
In northwest Argentina, one of the main limiting factors for production of tomato and pepper crops is the attack of whitefly, whose population dynamics is poorly known. Thus, the aim of this study was to determine the abundance and population dynamics of B. tabaci in greenhouse tomato and pepper crops in Tucumán Province.
The study was carried out in Lules department (26º55'60" S 65º20'60" O; 382 AMSL), Tucumán province, Argentina. This area is part of the humid and per humid piedmont agrological region, whose main characteristic is its high soil fertility due to the presence of Lules river alluvial fan (Zuccardi and Fadda, 1992).
Sampling took place from July 2008 to March 2009 in four crop plots with Almeria type cover: two Temporada variety tomato crops (I and II) and two pepper crops APL-82 variety (III and IV), both under conventional pest management, but with rational use of insecticides: in tomato BIO SPAN was used, while pepper crops were treated with Lamdex. Plots I and II contained 2.200 and 2.180 plants respectively. Plots III and IV contained 2.080 and 2.140 pepper plants respectively. The whole production cycle of both crops was recorded. Adults were sampled using yellow sticky traps 5 x 7 cm, replaced every 15 days. For their placement, the number of plants and rows of each plot was considered, and a diagonal pattern was followed, including the extremes and center of evaluated area. The immature forms (eggs, nymphs, and pupae) were collected fortnightly from the top, medium, and lower strata, extracting one leaflet per strata, established according to the size of each plot, and to the total number of plants. The sampling was carried out following the criterion of Bueno et al. (2005) to encompass all the developmental stages of B. tabaci. Subsequently, the leaflets were placed in individual plastic bags and transferred in cases to the Laboratory of Agricultural Zoology of the Estación Experimental Agroindustrial Obispo Colombres (EEAOC) (Las Talitas, Tucumán). The identification and quantification of the immature and adult stages was performed through stereoscopic microscopes following specific keys (Caballero, 1994, 1996; Caballero and Pitty, 1995), and the data was recorded into a spreadsheet where the date, plant number, stratum, crop, and producer were registered for immature forms; and the date, place, crop, producer, and trap number were registered for adults.
The relative abundance (%) of the different developmental stages of B. tabaci by crop type and sampled plot was determined, calculated as the abundance of each of stages in relation with total abundance of collected specimens. Aditionally, population fluctuation of the different developmental stages throughout the sampling period was determined, obtaining abundance variation graphs by crop type and plot. Prior to the analyses, abundance values were transformed logarithmically (ln (n + 1)), to improve data visibility. Finally, Spearman non-parametric correlation analyses were performed, using InfoStat statistic software (INFOSTAT, 2007), to determine the relation between number of eggs and number of adults.
A total of 121.075 specimens of B. tabaci were collected, of which 12.630 corresponded to eggs, 99.465 to adults, 8.718 to nymphs, and 262 to pupae. In tomato plot I, a higher number of adults (92.4 %) was recorded, followed by nymphs (5.6 %), eggs (1.7 %), and pupae (0.2 %). In Plot II, a higher abundance of adults (89.5 %) was also registered, followed by eggs (6.1 %), nymphs (4.2 %), and pupae (0.2 %). In pepper plot III, a higher number of adults (79.5 %) was also found, followed by eggs (12.8 %), nymphs (7.5 %), and pupae (0.2 %) and in plot IV a higher number of adults (75.7 %) was observed as well, followed by nymphs (13.1 %), eggs (11.1 %), and pupae (0.1 %).
In general, the behavior of the different developmental stages of B. tabaci by plot showed variations throughout the sampling period, and a population increase in the two final weeks was observed. In plot I, B. tabaci showed regular fluctuations throughout the sampling period, with egg and nymph abundance peaks mainly by week 11 and 13 (April, 2009); and two abundance peaks in adults, one during week 5 (March, 2009) and another in week 13 (April, 2009). Furthermore, week 13 had the highest number of specimens (figure 1 a y b).
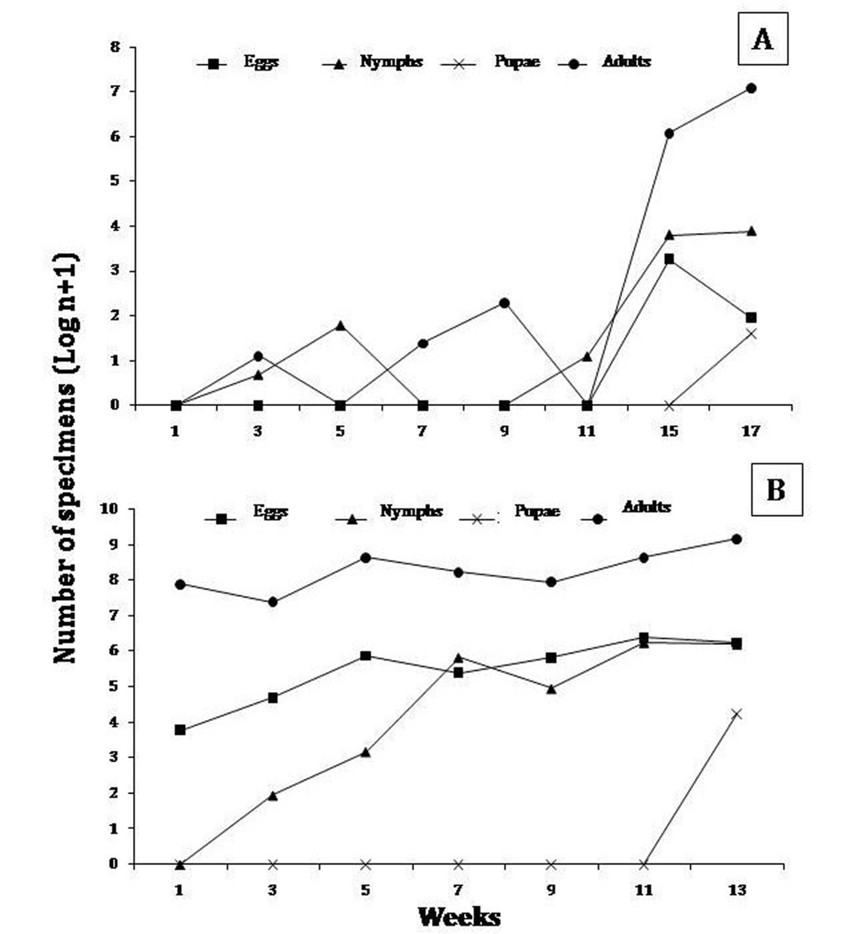
The population dynamics of B. tabaci in pepper crops showed a similar pattern of behavior to that observed in tomato crops. In plot III, B. tabaci fluctuated regularly, with an increase in the number of eggs and nymphs mainly by week 13 (January, 2008), followed by week 22 (March, 2009); and an increase in the number of adults by week 13 (January, 2008), and 18 (February, 2008). In plot IV, the different developmental stages of B. tabaci also showed regular distribution patterns, with an increase in number of eggs during week 27 (November, 2008), followed by an increase in number of nymphs by week 25 (November, 2008). In turn, adult specimens showed abundance peaks in week 27 (November, 2008) (figure 2 a y b).
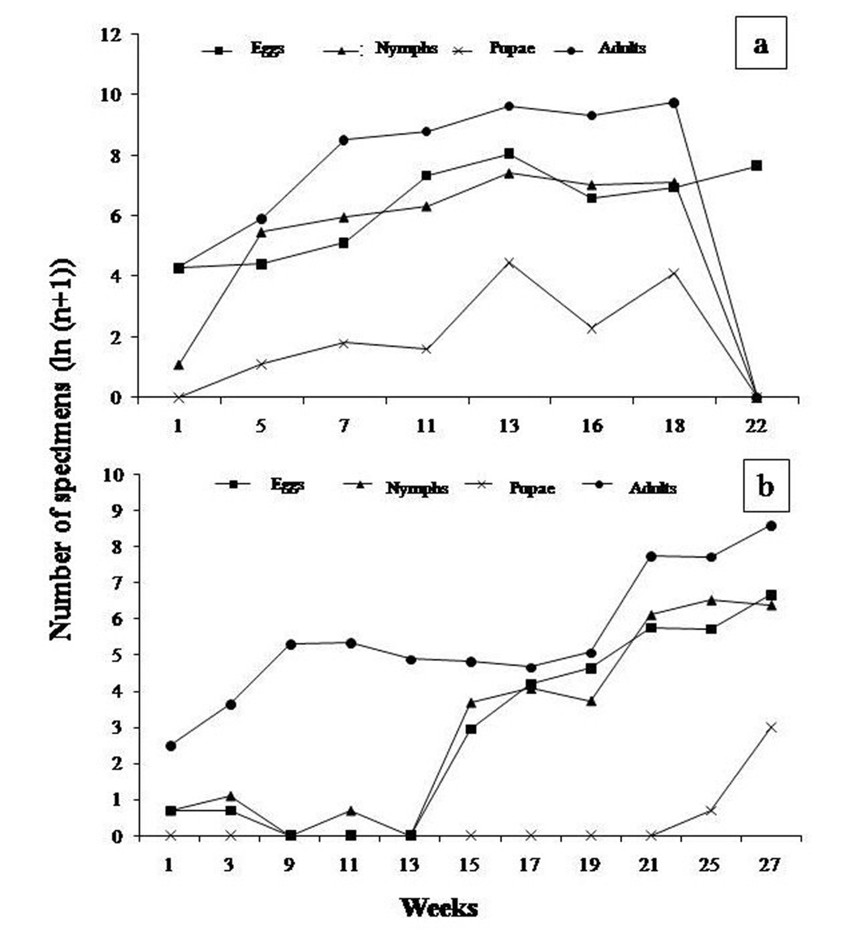
It is worth mentioning that exploratory sampling of B. tabaci in the field was performed, in which a higher number of nymphs and adults was registered, and irregular fluctuations were found, with an increase in adult populations by week 6 (October, 2008). When comparing these findings with those observed in under cover crops, it can be suggested that B. tabaci populations behave differently, which might be due to the different temperature, humidity, and photoperiod conditions, which are controlled inside greenhouses.
Finally, the correlation analysis between the number of eggs and the number of adults of B. tabaci was significant both in tomato crops (r = 0.94; p < 0.01) and in pepper crops (r = 0.87; p < 0.01). Thus, B. tabaci adult sampling in under cover plots can give an approximation of crop egg density, which is in itself an important tool for decisionmaking aiming at controlling whiteflies in the region.
According to these results, we could say that the relative abundance of Bemisia tabaci differed according to the developmental stage, crop type, plot, and sampling week; and a higher abundance of B. tabaci was found in pepper crops. Certain studies report the existence of B. tabaci oviposition and nymph development preferences over plants with pubescent leaves, such as the tomato (Morales and Cermeli, 2001; Sánchez et al., 1997). However, in these studies it was observed that B. tabaci took longer to develop in tomato crops in relation to other hots plants (Phaseolus vulgaris L., Gossypium hirsutum L., Hibiscus rosa-sinensis L. y Euporbia pulcherrima Willd.).
Overall, the fluctuation of B. tabaci was regular in both crop types, and population increases occurred gradually since infestation, with a higher abundance of adults towards the last weeks of sampling. These results agree with those observed in populations of immature stages of B. tabaci in Almería, Spain where a gradual increase towards the end of the greenhouse pepper crop period was observed, and where adult populations exhibited three abundance peaks that might have been due to different generations (González Zamora and Moreno Vázquez, 1996). Likewise, in Maracaibo, Venezuela, under abiotic controlled conditions it was determined that B. tabaci can present 13 to 14 generations per year in tomato crops, and approximately four to five cycles in host plants in 120 days (Sánchez et al., 1997).
Acknowledgments
We would like to thank the Agricultural Zoological Section of the Estación Experimental Agroindustrial Obispo Colombres (EEAOC), Las Talitas, Tucumán, and especially give thanks to Eduardo Willink, Director of Special Disciplines.
References
Brown, J. 1993. Evaluación crítica sobre los biotipos de mosca blanca en América de 1989 a 1992. In: Hilje, L., Arboleda, O. Editor. Las moscas blancas (Homoptera: Aleyrodidae) en América Central y el Caribe. Informe Técnico nº 205. CATIE, Turrialba.
Bueno, J.M., Cardona, C. and Chacón, P. 2005. Fenología, distribución y desarrollo de método de muestreo para Trialeurodes vaporariorum (Westwood) (Hemiptera: Aleyrodidae) en habichuela y fríjol (Phaseolus vulgaris L.). Revista Colombiana de Entomología 31 (2): 161-170.
Byrne, N., Bellows, T.S.Jr. and Parrella, M. 1990. ‘Whiteflies in agricultural systems’. In: Gerling, D. Editor. Whiteflies: their bionomics, pest status and management. Intercept L.T.D., Andover.
Caballero, R. 1994. Calve de campo para inmaduros de mosca blanca de Centroamérica (Homóptera: Aleyrodidae). Revista Ceiba 35 (1): 47-51.
Caballero, R. 1996. Identificación de moscas blancas. L. Hilje Metodologías para el estudio y manejo de moscas blancas y geminivirus. CATIE, Turrialba.
Caballero, R. and Pitty, A. 1995. IV Taller Latinoamericano sobre moscas blancas y geminivirus. Revista Ceiba 36: 1.
García Marí, F., Costa Comelles, J. and Ferragut Perez, F. 1994. Plagas Agrícolas. Phytoma España 58: 63-72.
González Zamora, J. and Moreno Vázquez, R. 1996. Análisis de las tendencias poblacionales de Bemisia tabaci (Gennadius) (Homoptera: Aleyrodidae) en pimiento bajo plástico en Almería. Boletín Sanidad Vegetal Plagas 22: 159-167.
INFOSTAT. 2007. Infostat software estadístico, versión 2007. Facultad de Ciencias Agropecuarias, Universidad Nacional de Córdoba, Argentina: Grupo InfoStat.
López-Ávila, A. 2005. Biología y control: control biológico de las moscas blancas. Taller. Centro de Investigación Tibaitatá, Bogotá.
Lourenção, A.L. and Nagai, H. 1994. Surtos populacionais de Bemisia tabaci no estado de São Paulo. Bragantia 53 (1): 53-59.
Llorens Climent, J. and Garrido Vivas, A. 1992. Homoptera III. Moscas Blancas y su Control Biológico. PISA, Alicante.
Mound, L. and Halsey, S. 1978. Whitefly of the World. A systematic catalogue of the Aleyrodidae (Homoptera) with host pant and natural enemy. British Museum (Natural History), London, UK.
Morales, P. and Cermeli, M. 2001. Evaluación de la preferencia de la mosca blanca Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) en cinco cultivos agrícolas. Entomotropica 16 (2): 73-78.
Naranjo, S., Ellsworth, P. and Hagler, J. 2004. Conservation of natural enemies in cotton: role of insect growth regulators for management of Bemisia tabaci. Biological Control 30: 52-72.
Polack, A. 2005. Manejo integrado de moscas blancas. Boletín Hortícola: Protección Vegetal. Instituto Nacional de Tecnología Agropecuaria, INTA San Pedro. Buenos Aires.
Salguero, V. 1993. Perspectiva para el manejo del complejo mosca blanca-virosis. In: Hilje, L. and Arboleda, O. Editor. Las moscas blancas (Homoptera: Aleyrodidae) en América Central y el Caribe. Serie Técnica. Informe Técnico nº 205. CATIE, Turrialba.
Sánchez, A., Gerud-Pouey, G. and Esparza, D. 1997. Biología de la mosca blanca del tabaco, Bemisia tabaci (Homoptera: Aleyrodidae) y potencial para desarrollar sus poblaciones sobre cinco especies de plantas hospederas. Revista Facultad de Agronomía 14: 193-206.
Vet, L., Van Lenteren, J. and Woets, J. 1980. The parasite-host relationship between Encarsia formosa (Hymenoptera: Aphelinidae) and Trialeurodes vaporariorum (Homoptera: Aleyrodidae). IX. A review of the biological control of the greenhouse whitefly with suggestions for future research. Journal of Applied Entomology 90: 26-51.
Viscarret, M. 2000. Estudios biológicos sobre Aleyrodidae de importancia económica (Insecta: Hemiptera) con énfasis en el complejo Bemisia tabaci (Gennadius) y su posible control biológico. Doctoral Thesis. Universidad Nacional de Buenos Aires, Buenos Aires, Argentina.
Viscarret, M., Lopez S.N. and Botto, E. 2001. Estudios fitotóxicos y de tabla de vida y fecundidad sobre el biotipo ARG1 del complejo Bemisia tabaci (Hemiptera: Aleyrodidade). Revista de la Sociedad Entomológica Argentina 60 (1-4): 167-176.
Zuccardi, R. and Fadda, G. 1992. Bosquejo agrológico de la provincia de Tucumán. Miscelánea Nº 86. Facultad de Agronomía y Zootecnia – UNT, Argentina.
How to cite: Ortega, E.S., Veggiani-Ayba, C.A., Ávila, A.L. and Reguilón, C. Preliminary study of the fluctuation of Bemisia tabaci (Hemiptera: Aleyrodidae) in greenhouse tomato and pepper crops, Tucumán, Argentina. Intropica 14(1): 60-64. DOI: http://dx.doi.org/10.21676/23897864.2766.